Technology has improved at a rapid pace over recent decades – shouldn’t your TMS system as well?
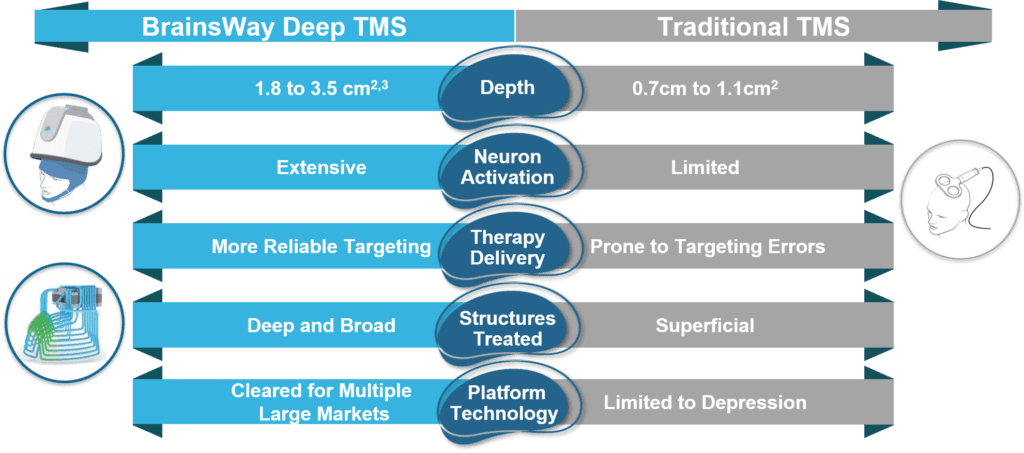
BrainsWay Deep TMS™ is the most advanced TMS technology available, with a number of key advantages over Traditional TMS systems.
Through unparalleled innovation, clinical research, and use in hundreds of clinics and hospitals throughout the world, only BrainsWay is advancing Deep TMS into new therapy areas in psychiatry, neurology, and addiction medicine.
BrainsWay’s unique coil design enables deeper and broader stimulation than traditional, figure-8 TMS coils, resulting in greater activation of neurons, less likelihood of targeting errors, and high efficacy rates1. Traditional TMS coils have changed minimally since first used in 1985.
Clinical advantages of Deep TMS over traditional TMS have been validated through an independent head-to-head study of TMS technologies1.
Beyond the technology itself, BrainsWay empowers mental health providers to be successful in treating patients with TMS via our flexible business models and total customer support.
BrainsWay is leading the development of novel treatment for a broad range of brain disorders.
Unlike traditional TMS systems that focus mainly on treating Major Depressive Disorder (MDD), BrainsWay Deep TMS is the only TMS device with peer-reviewed clinical data demonstrating efficacy for MDD, Anxious Depression, Late-Life Depression, OCD, and Smoking Addiction.
BrainsWay also continues to invest in research to potentially expand applications of Deep TMS. Our products have been CE-mark for additional psychiatric and neurological disorders in Europe, although they are not FDA-cleared or available for such uses in the U.S.
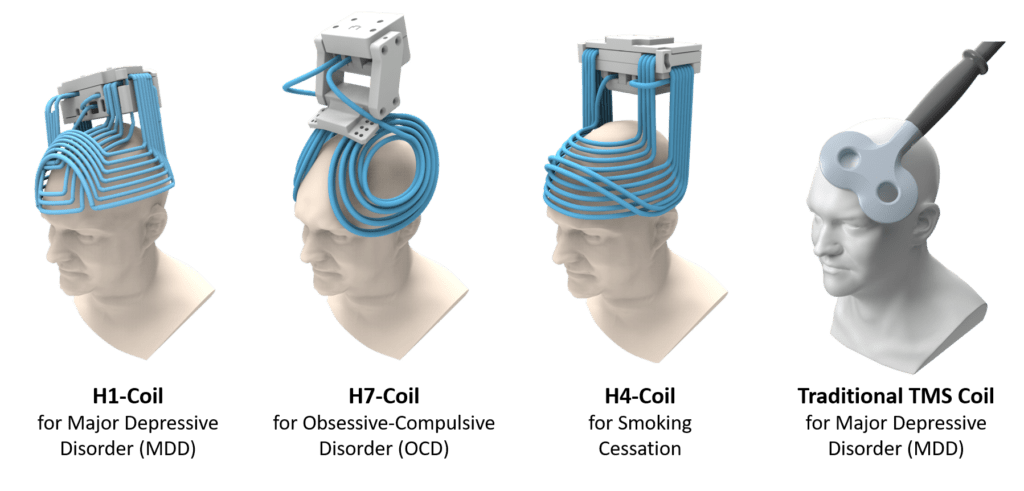
BrainsWay Deep TMS has been validated in 60+ randomized controlled trials (RCTs) with sham (placebo) involving over 3,000 patients and is currently being evaluated in 10+ ongoing RCTs. Research on Deep TMS has led to 300+ publications. No traditional TMS company has committed to this level of study of their technology.
In everyday use, nearly 600 BrainsWay systems are in operation across North America, and approximately 100,000 patients have been treated in over 2.5 million treatment sessions2.
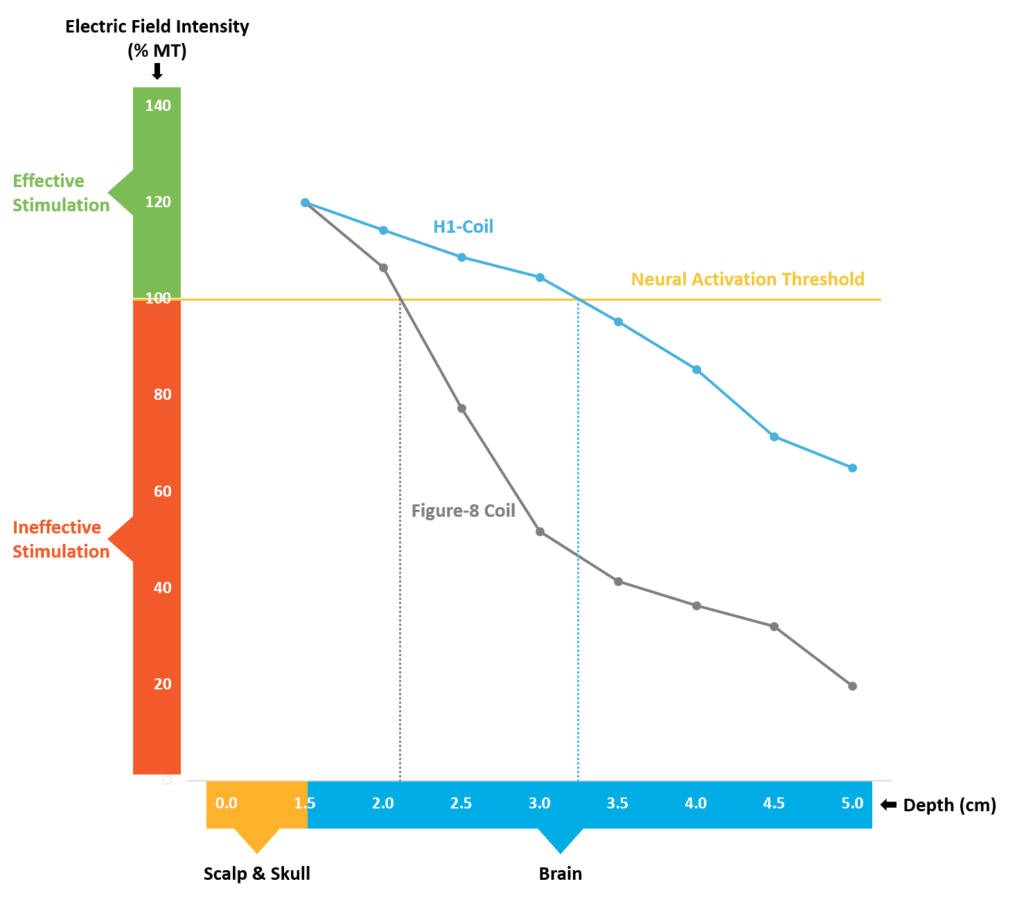
Deep TMS technology, unique to BrainsWay, enables non-invasive activation of deeper and broader brain structures than traditional figure-8 coil TMS systems, allowing the H-Coil to potentially address a wider range of neuropsychiatric conditions.
With all forms of TMS, the intensity of stimulation naturally decays as it reaches deeper into the brain. Our unique coil design allows for the strength of stimulation to be maintained at effective levels even in regions deeper within the brain that are affiliated with the indications we treat1.
For patients that are older, traditional TMS may not penetrate deep enough to generate a therapeutic effect, as in the case of late-life depression3.
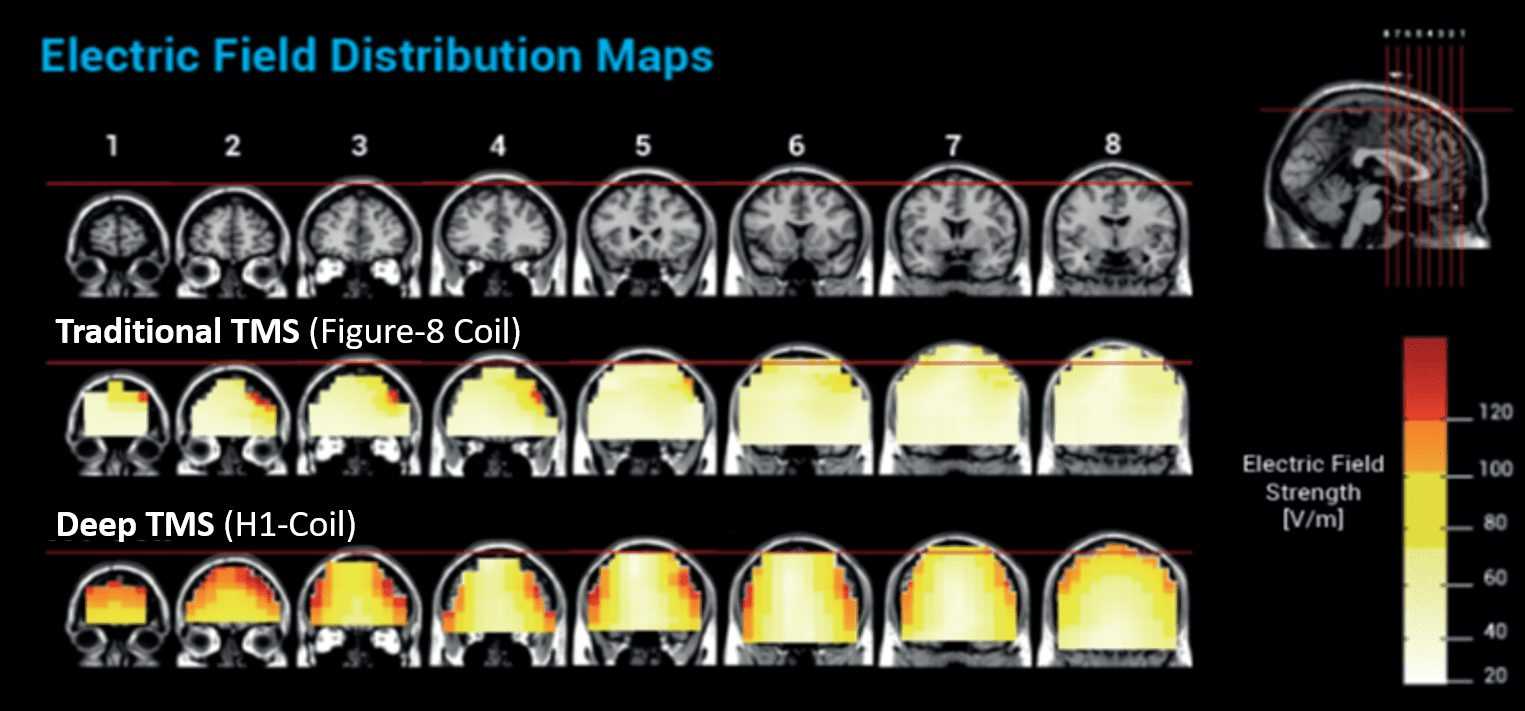
The larger electric field generated by the H-Coil stimulates significantly more neurons, reducing the likelihood that the target brain region is missed. With traditional TMS, if the positioning is off by as little as 1mm, 40% of the required dosage can be lost4. BrainsWay’s reduced likelihood of missing the target avoids the need for a pricey, complex MRI-based neuro-navigation system.
The H-Coil is integrated into BrainsWay’s cushioned helmet, which is flexibly designed to allow for steady, consistent contact with the patient’s scalp throughout the course of treatment, rendering contact sensing software unnecessary. Unlike other traditional TMS systems, the H-Coil is secured to the patients head, reducing the potential for movement of the coil during TMS therapy – essential to ensure reliable clinical outcomes.
 |
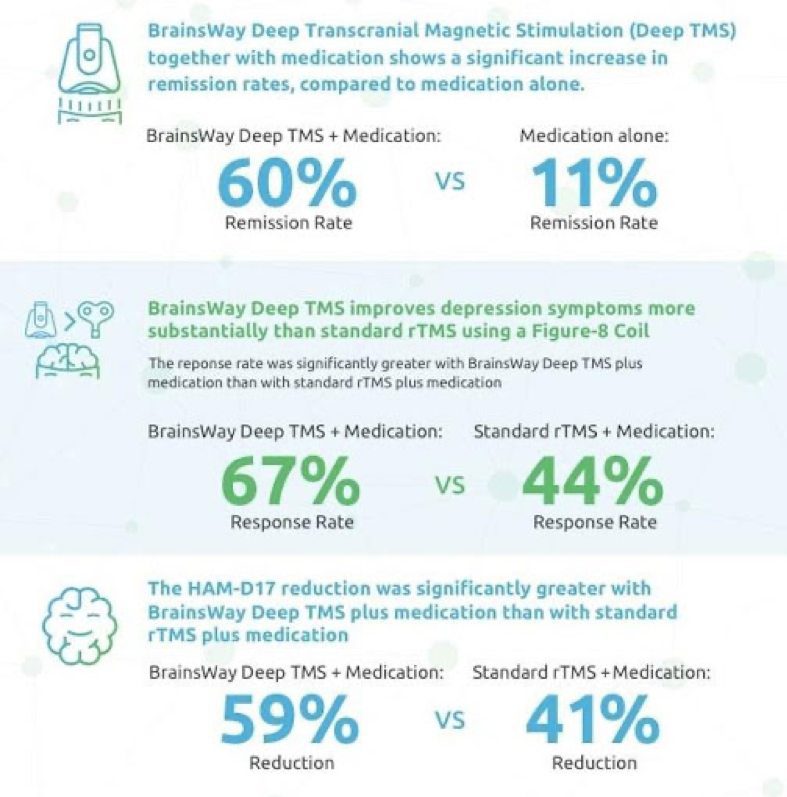
The only independent, head-to-head study of TMS technologies, published in the Journal of Psychiatric Research, determined that Deep TMS plus standard medication was significantly more effective at reducing depression levels among Major Depressive Disorder (MDD) patients compared with Traditional TMS* plus medication or medication alone5.
Deep TMS had no difference in safety or tolerability from Traditional TMS.
*The Magstim® Rapid² device was used in this study.
(Magstim Company, Spring Gardens, UK)
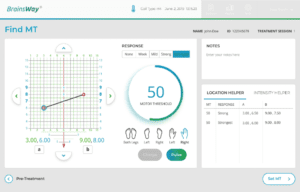
BrainsWay’s touchscreen interface is designed to be user-friendly and guides the operator through the process of determining a patient’s motor threshold intensity and coil positioning. This innovative feature allows the setup process for each patient to be quicker than other traditional TMS systems.
With a traditional TMS system, the clinic is restricted to using a specific chair. The patient’s head is immobilized. Any movement, even a 1mm shift, could result in missing the target.
BrainsWay’s H-Coils are integrated into a cushioned, cooled helmet, allowing the patient to retain mobility during the treatment without compromising treatment accuracy. Clinics can select any chair they deem comfortable and in keeping with the clinic’s aesthetic. The flexibility also allows for patients with mobility issues, such as those in wheelchairs.