A significant unmet need exists in the treatment of mental health and addiction conditions. Current treatment options, such as pharmacotherapy, psychotherapy, and electroconvulsive therapy, are not sufficient for a substantial population of patients.
BrainsWay’s patented breakthrough treatment, Deep Transcranial Magnetic Stimulation (Deep TMS™), offers a new treatment option using cutting-edge neuroscience.
Deep TMS is a clinically proven, noninvasive in-office brain stimulation treatment that uses magnetic fields to activate neural networks in the brain to improve symptoms of mental health and addiction conditions, including depression, anxious depression, late-life depression, obsessive-compulsive disorder, and smoking addiction.
Deep TMS uses flexible H-Coils embedded in a patient and operator-friendly helmet to noninvasively activate targeted brain structures. Our patented H-Coil design enables deeper and broader stimulation than traditional TMS coils, resulting in greater activation of neurons, less likelihood of targeting errors, and powerful efficacy.

The H1 Coil stimulates the bilateral prefrontal cortex, with a preference for the left dorsolateral prefrontal cortex. In addition to numerous published, double-blind, randomized controlled trials validating the efficacy of Deep TMS for depression, clinical data of over 1,000 patients in real practice settings has shown compelling results. Among patients who completed at least 30 sessions, approximately 4 in 5 achieved response and approximately 2 in 3 achieved remission.

The H7 Coil stimulates the anterior cingulate cortex and the medial prefrontal cortex. Complementing a large scale, double-blind, multicenter randomized controlled trial, which demonstrated strong response rates, greater than 1 in 2 OCD patients who completed 29 sessions in real clinical practice achieved a sustained response.

The H4 Coil stimulates the bilateral insula and prefrontal cortex. Nearly 1 in 3 patients that completed 18 sessions achieved 4 or more weeks of abstinence from smoking in a large scale, double-blind, multicenter randomized controlled trial. Two out of 3 patients who quit smoking by the end of treatment remained abstinent for at least an additional three months.
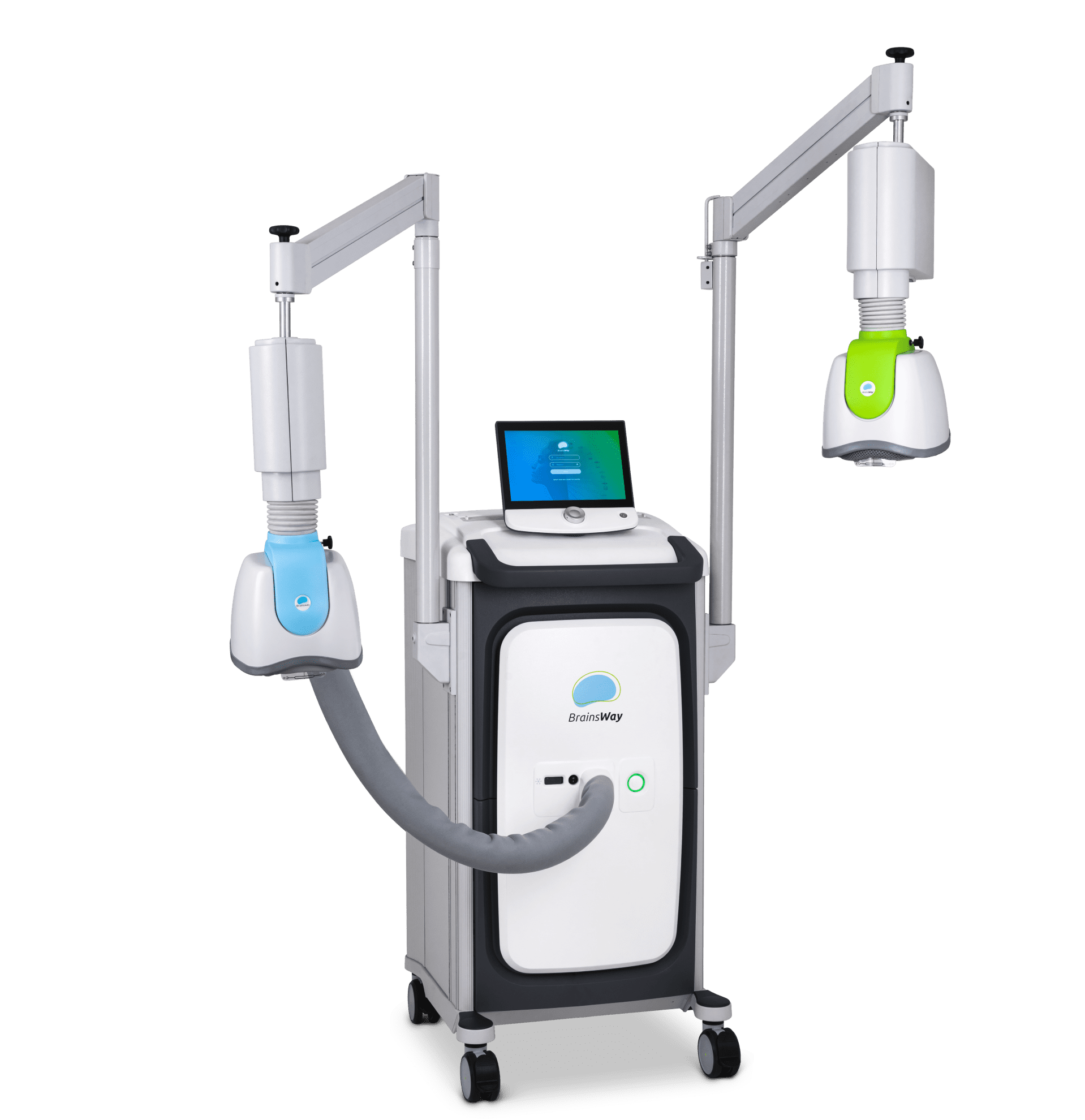
BrainsWay’s superior science is demonstrated in our innovative Deep TMS System, enabling greater ease-of-use for operators and superior patient comfort.
BrainsWay’s patented H-Coils are integrated into cushioned, cooled helmets, allowing the patient to retain mobility during treatment without compromising treatment accuracy.
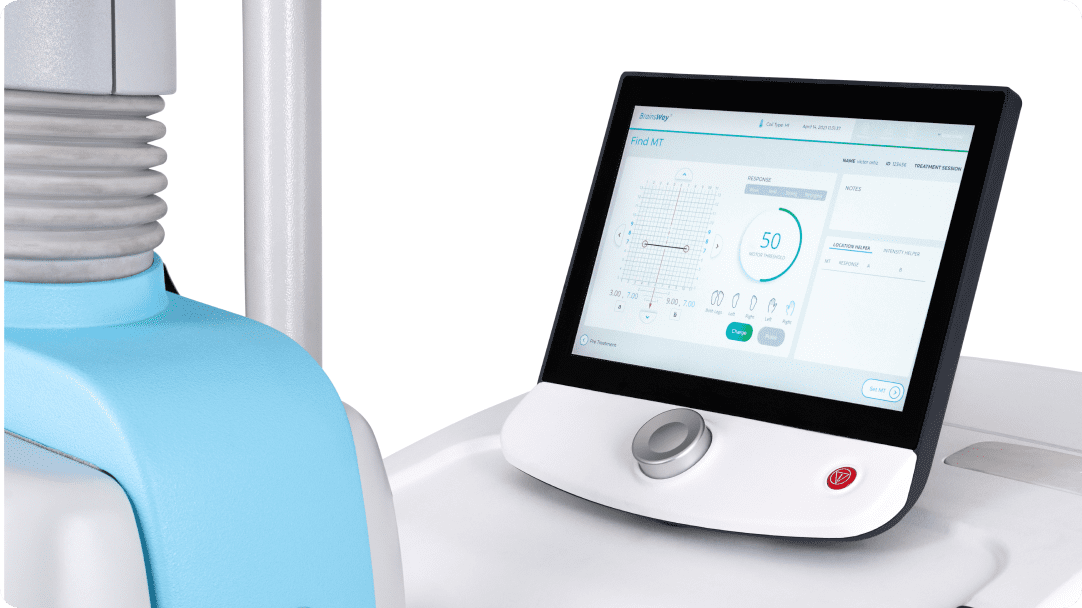
Built-in FDA protocols and guided Motor Threshold mapping result in quick patient setup. An advanced patient management system allows the clinic to understand system usage and patient outcomes.

Our luxurious patient cap pairs an intuitive grid system with a targeting guide on the helmet to simplify positioning and movement of the coil for the patient’s specific brain landmarks. No rulers or pencils necessary.
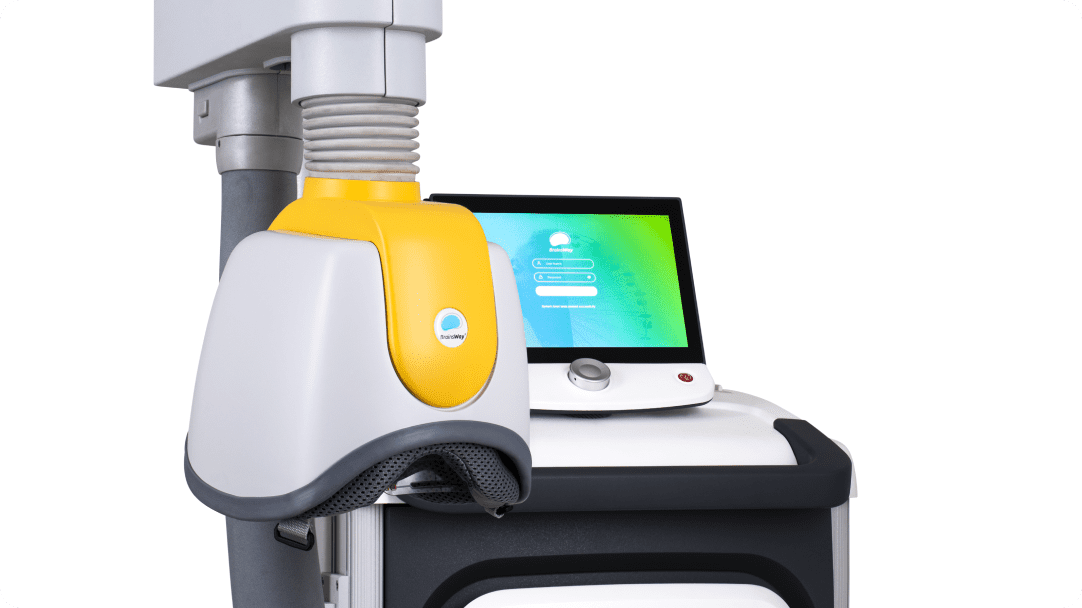
Each coil is connected to a positioning arm that allows rotation of the helmet in all dimensions, enabling proper positioning on the patient’s head. This flexibility also allows the clinic to use any treatment chair comfortable for the patient and in keeping with the clinic’s design aesthetic.
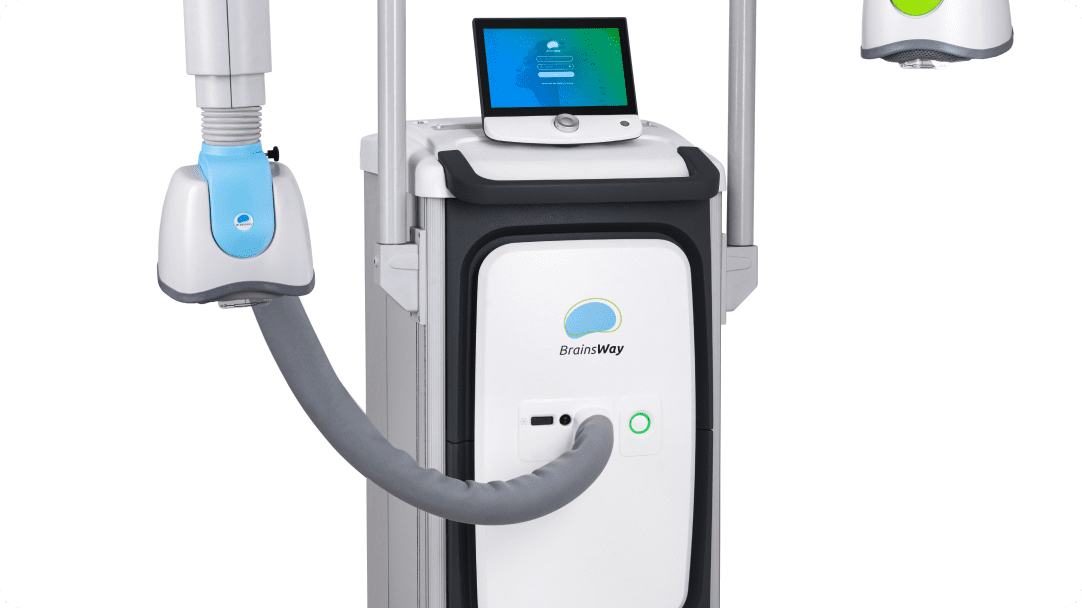
The system stimulator and power supply generate electromagnetic pulses with controlled output, frequency, and duration for reliable performance.

By continuously streaming air to the helmet, the system maintains ambient temperature in the coils for repeated operation, while enhancing patient comfort.

The foot pedal allows the operator to initiate or cease pulses in place of using the touchscreen for extra convenience and safety.
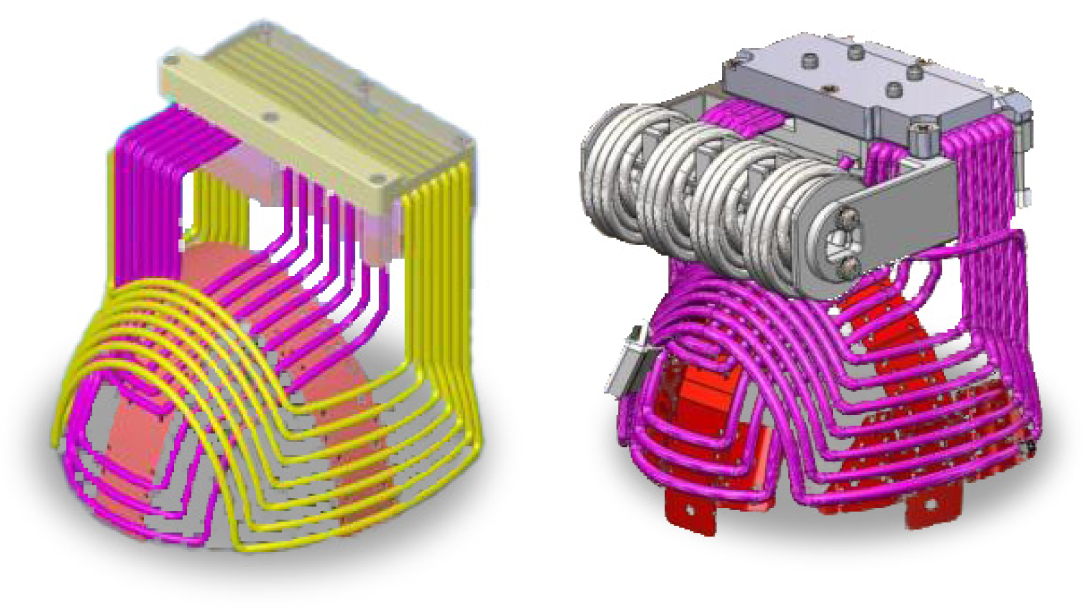
Clinical research conducted with the BrainsWay Deep TMS System is effectively blinded using a sham coil integrated in the helmet with the treatment coil. To the patient and operator, the helmet appears the same, pulses sound the same, and stimulation feels the same on the scalp, resulting in clinical studies that can truly validate the efficacy of Deep TMS treatment.