*Note, the numbers contained herein reflect certain updates to the previous unaudited financial results for the first
Quarter 2021, pursuant to BrainsWay’s May 24, 2021 filing with the SEC, available free of charge at
http://www.sec.gov
Strong Revenue Growth of 47% Year-over-Year in Q1 2021
Successfully Executed Initial U.S. Controlled Market Release of Deep TMS for Smoking
Addiction
Strengthened Balance Sheet Through Successful Equity Offering; Ended Q1 2021 with $58.5
Million in Cash
CRESSKILL, N.J. and JERUSALEM, Israel, May 20, 2021 (GLOBE NEWSWIRE) – BrainsWay Ltd. (NASDAQ & TASE: BWAY) (“BrainsWay” or the “Company”), a global leader in advanced noninvasive neurostimulation treatments for mental health disorders, today reported first quarter 2021 financial results and provided an operational update.
Recent Financial and Operational Highlights
“We are extremely pleased with the overall trends in our business, and are working to continue the strong momentum achieved late in 2020 and during the first quarter throughout the year,” stated Christopher von Jako, Ph.D., President and Chief Executive Officer of BrainsWay. “Our revenues of $6.1 million represented a significant 47% increase over our first quarter of 2020, demonstrating the resiliency of our business and continued emergence from the COVID-19 pandemic. We marked a true milestone for our revolutionary approach to mental health treatment with the recent treatment of our 100,000th patient, and this was complemented by impressive commercial and regulatory achievements, including our successful follow-on raise of $45.2 million in gross proceeds, the controlled market release of our smoking addiction system, and the FDA’s clearance of our three-minute Theta Burst depression protocol. We believe these accomplishments position us well to generate long-term shareholder value and to advance in our mission to improve health and transform lives.”
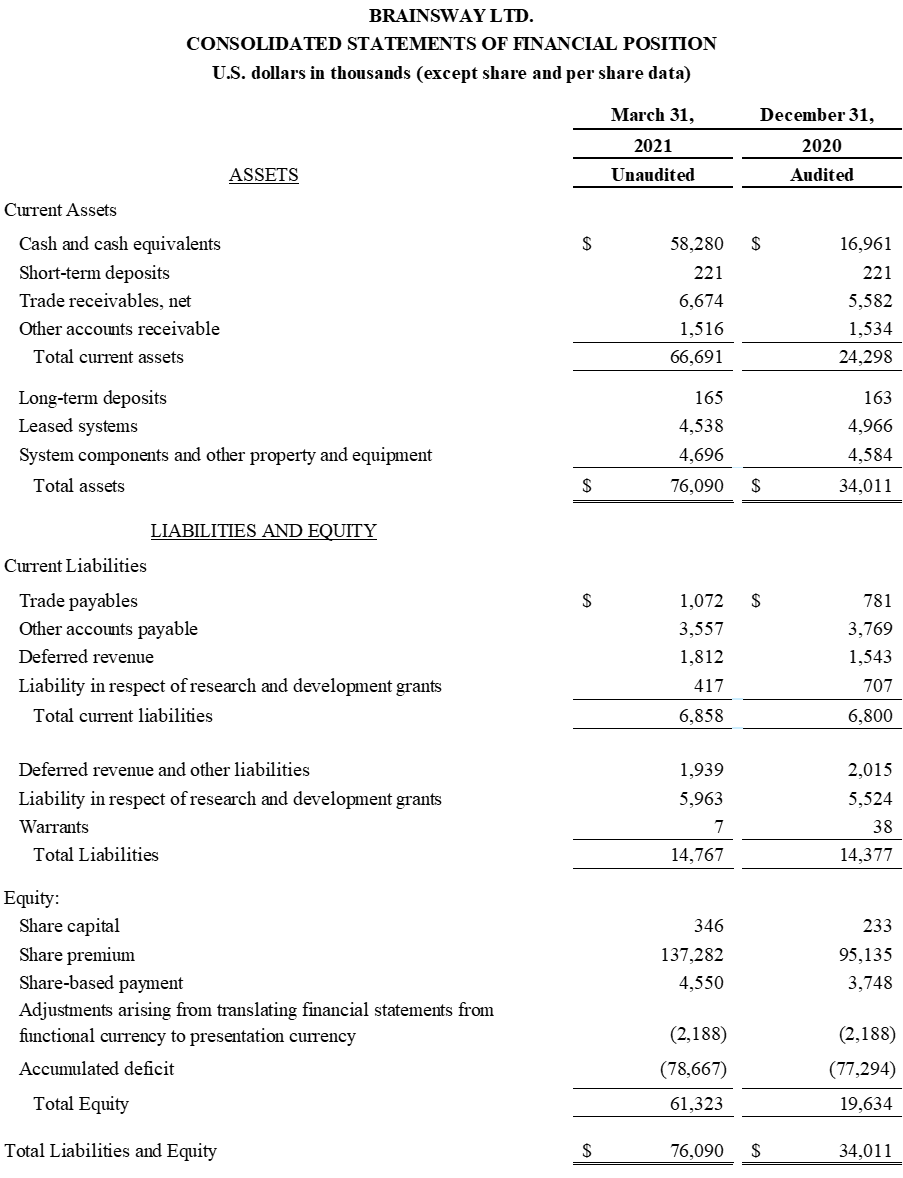
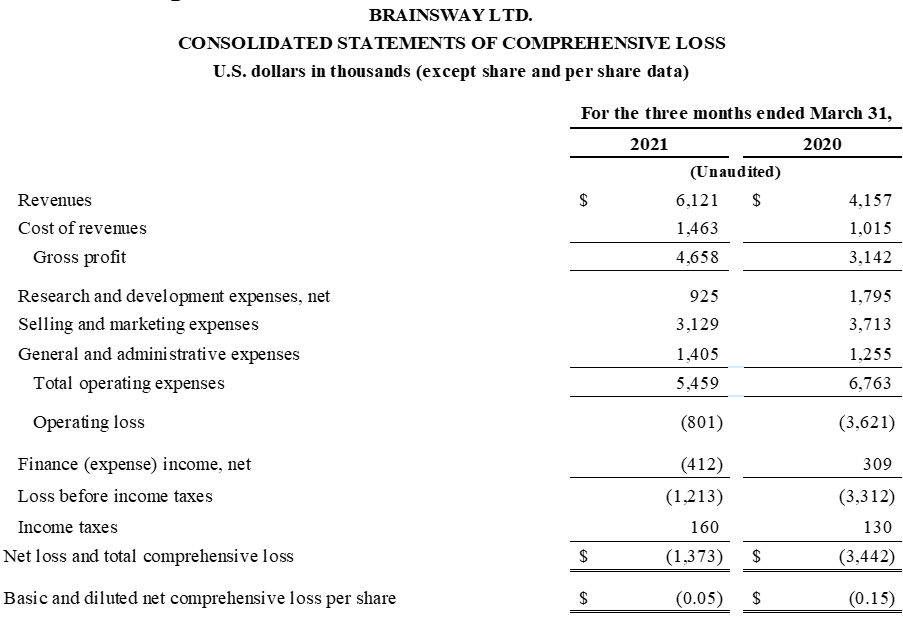
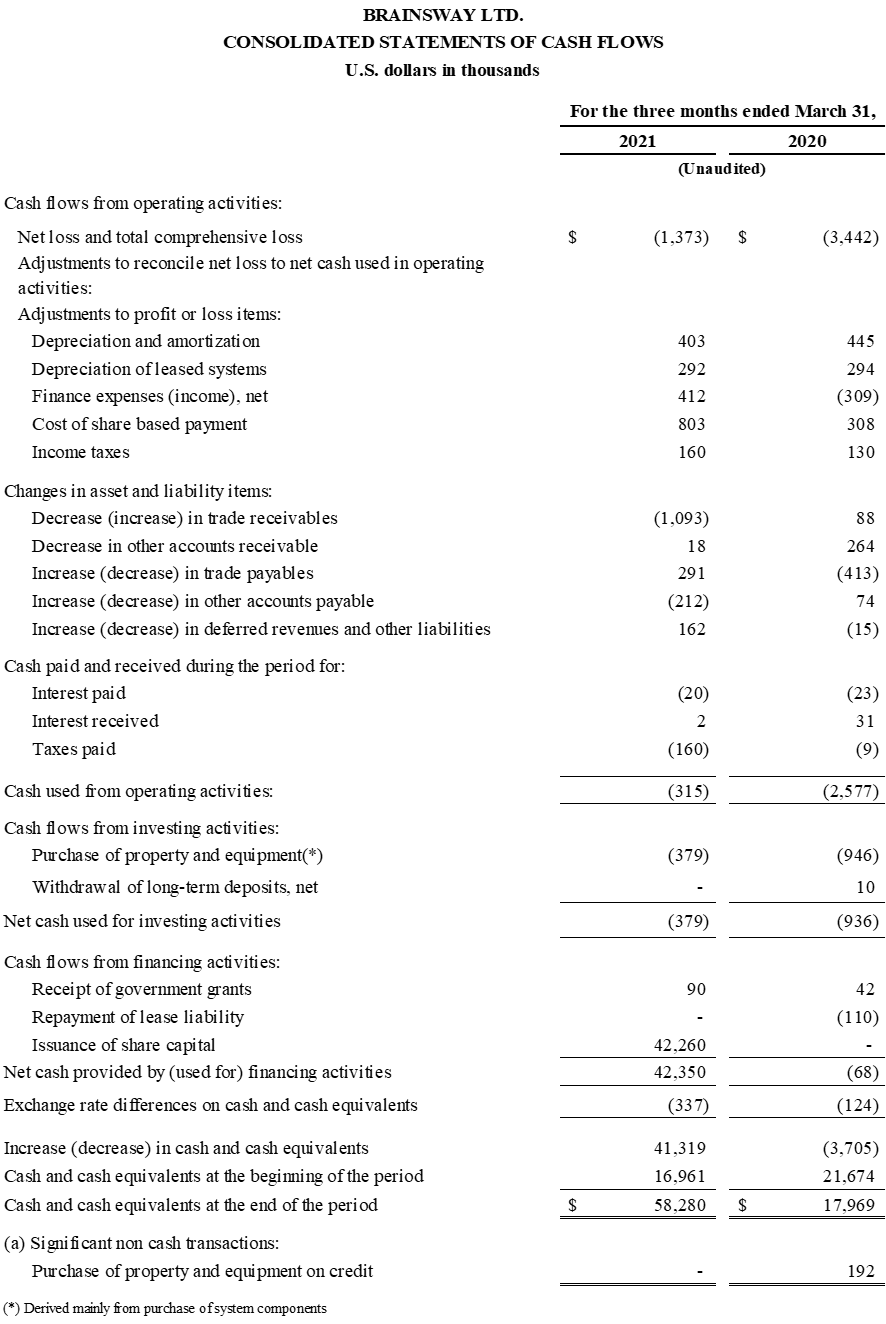
First Quarter 2021 Financial Results
About BrainsWay
BrainsWay is a global leader in advanced noninvasive neurostimulation treatments for mental health disorders. The Company is boldly advancing neuroscience with its proprietary Deep Transcranial Magnetic Stimulation (Deep TMS) platform technology to improve health and transform lives. BrainsWay is the first and only TMS company to obtain three FDA-cleared indications backed by pivotal studies demonstrating clinically proven efficacy. Current indications include major depressive disorder, obsessive-compulsive disorder, and smoking addiction. The Company is dedicated to leading through superior science and building on its unparalleled body of clinical evidence. Additional clinical trials of Deep TMS in various psychiatric, neurological, and addiction disorders are underway. Founded in 2003, with offices in Cresskill, NJ and Jerusalem, Israel, BrainsWay is committed to increasing global awareness and broad access to Deep TMS. For the latest news and information about BrainsWay, please visit www.brainsway.com.
Forward Looking Statements
This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements may be preceded by the words “intends,” “may,” “will,” “plans,” “expects,” “anticipates,” “projects,” “predicts,” “estimates,” “aims,” “believes,” “hopes,” “potential” or similar words. These forward-looking statements and their implications are based on the current expectations of the management of the Company only and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. The following factors, among others, could cause actual results to differ materially from those described in the forward-looking statements: inadequacy of financial resources to meet future capital requirements; changes in technology and market requirements; delays or obstacles in launching and/or successfully completing planned studies and clinical trials; failure to obtain approvals by regulatory agencies on the Company’s anticipated timeframe, or at all; inability to retain or attract key employees whose knowledge is essential to the development of Deep TMS products; unforeseen difficulties with Deep TMS products and processes, and/or inability to develop necessary enhancements; unexpected costs related to Deep TMS products; failure to obtain and maintain adequate protection of the Company’s intellectual property, including intellectual property licensed to the Company; the potential for product liability; changes in legislation and applicable rules and regulations; unfavorable market perception and acceptance of Deep TMS technology; inadequate or delays in reimbursement from third-party payers, including insurance companies and Medicare; inability to commercialize Deep TMS, including internationally, by the Company or through third-party distributors; product development by competitors; inability to timely develop and introduce new technologies, products and applications, and the effect of the global COVID-19 health pandemic on our business and continued uncertainty and market impact relating thereto.
Any forward-looking statement in this press release speaks only as of the date of this press release. The Company undertakes no obligation to publicly update or review any forward- looking statement, whether as a result of new information, future developments or otherwise, except as may be required by any applicable securities laws. More detailed information about the risks and uncertainties affecting the Company is contained under the heading “Risk Factors” in the Company’s filings with the U.S. Securities and Exchange Commission, including the Company’s Annual Report on Form 20-F. Investors and security holders are urged to read these documents free of charge on the SEC’s web site at http://www.sec.gov.
Contacts:
BrainsWay:
Scott Areglado
SVP and Chief Financial Officer
Scott.Areglado@BrainsWay.com
Investors:
Bob Yedid
LifeSci Advisors
646-597-6989
Bob@LifeSciAdvisors.com